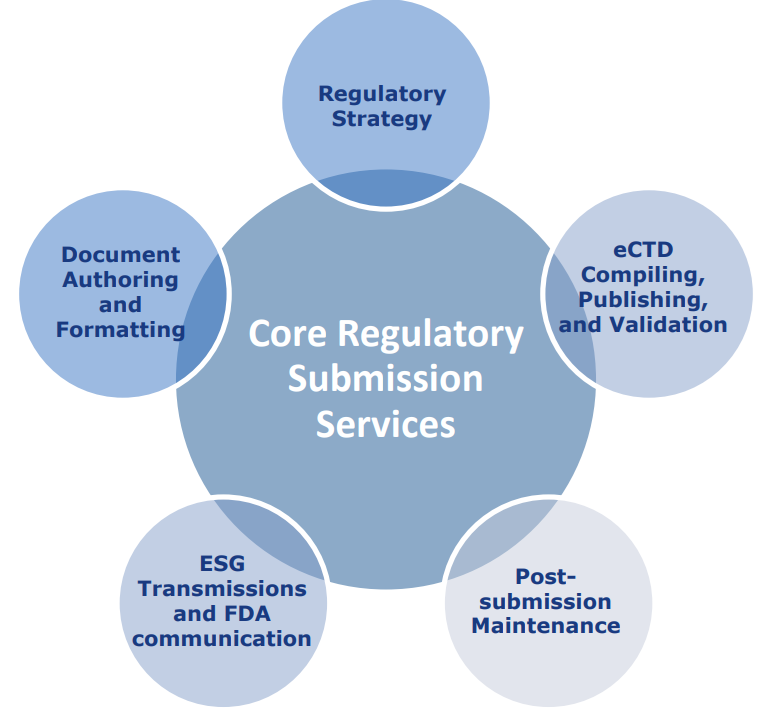
At DoubleBridge Technologies, we offer a full suite of eSubmission services tailored to meet your regulatory needs:
Seamless project management for regulatory submissions across all stages
Initial preparation, submission, and ongoing maintenance of INDs, NDAs, ANDAs, BLAs, and eDMFs
Specialized country-specific submission composition, validation, and publishing for FDA and EMA
Comprehensive formatting, editing, and hyperlink verification to ensure submission readiness
Expert preparation and organization of submission documents and cross references
Efficient preparation, publishing, and management of amendments and supplements
End-to-end document authoring and translation services to support global compliance
Secure and reliable submissions using our FDA-compliant company gateway
Access to our intuitive, cloud-based software portal for streamlined and timely submissions
Safe and compliant eCTD file storage and archival on our 21 CFR Part 11 secure servers